PARIS, 28-Apr-2022 — /EPR HEALTHCARE NEWS/ — ATTD 2022 – Diabeloop, pioneer in therapeutic artificial intelligence for diabetes management, presents today new results from a real-life cohort in Germany. The data show a constant improvement in Time In Range (70-180 mg/dL) combined with a significant reduction of hypoglycemia.
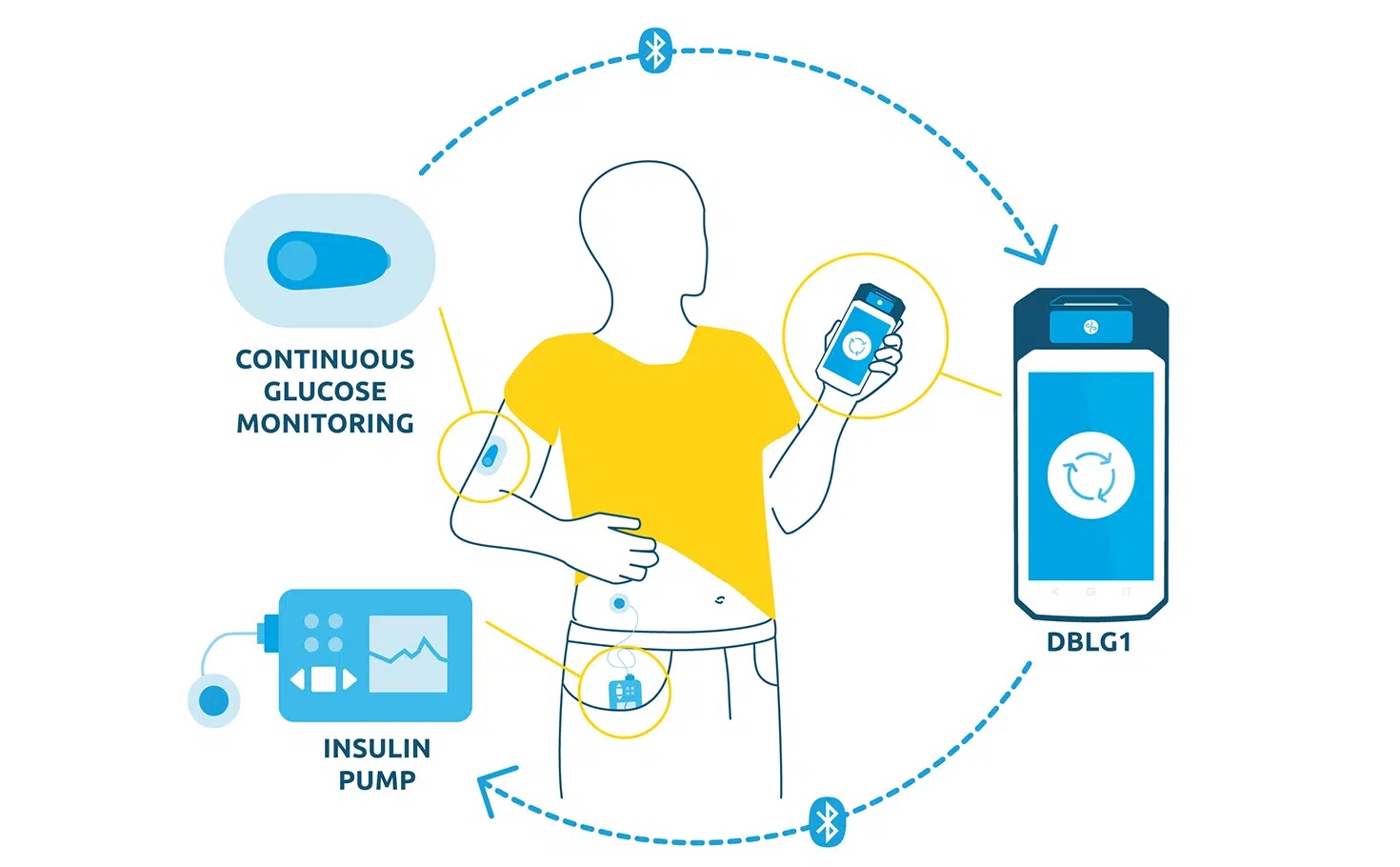
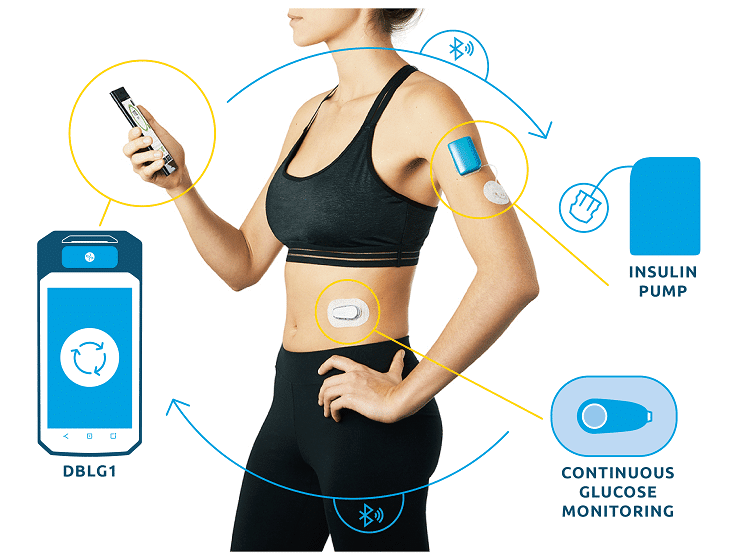
DBLG1, Diabeloop’s hybrid closed-loop, showed improvement of Time In Range and reduction of hypoglycemic episodes which allows for better diabetes management and improved quality of life.
Time In Range: a continuous improvement, with even more people equipped
Presented today at ATTD, the latest data from DBLG1¹ real-life cohort demonstrates an increase of 18.4 percentage point in Time In Range (TIR 70-180 mg/dL) on 974 patients.
This significant gain confirms the constant improvement in Time In Range, a key indicator of glucose control, in people living with Type 1 diabetes equipped with DBLG1 System since the WP7² pivotal trial (+12 points), the commercial pre-launch³ (+16.7 points) and the results of the first real-life cohort in Germany4 (+17.1 points).
With several thousand people equipped, Diabeloop’s solution continues to increase the Time In Range and thus improves the associated clinical benefits, such as better glucose control, less complications and improved quality of life.
These significant performances confirm the ability of Diabeloop’s solutions to improve glycemic control of people with T1D, in real-life, in a less controlled environment than in hospital supervised studies.
DBLG1 users in Germany, included in the real-life cohort, benefit from basic support at initialization. This demonstrates the ease of use of Diabeloop’s automated and personalized insulin delivery system and its interface.
Prof. Pierre-Yves Benhamou comments: “From the first study – WP7 which showed an increase in Time In Range of 12 points, to today with +18.4 points following the European commercial launch of DBLG1, the continuous improvement of the TIR is significant. The results observed on the new real-life cohort of nearly 1000 people in Germany are very impactful, with patients with a baseline TIR (70-180 mg/dL) of 54.6% reaching 73% TIR in closed-loop with DBLG1. The other very positive result observed in this cohort is the significant reduction in hypoglycemiaâ€.
A significant reduction of hypoglycemia combined with improvement in TIR
Associated with the increase in TIR, the retrospective data of the real-life DBLG1 cohort show a very significant reduction in time spent in hypoglycemia. Users of DBLG1 whose HbA1c was available at initiation spent only 0.9% of the time below 70 mg/dL and 0.1% of the time below 54 mg/dL5.
This variable has been constantly improving since the real-life WP72 study, which had already demonstrated a halving of the time spent below 70 mg/dL, a gain of more than 30 minutes less spent in hypoglycemia each day. More recently, the first real-life cohort in Germany demonstrated a time spent below 70 mg/dL at 1% and 0.2% below 54 mg/dL(4).
Thus, DBLG1 strongly contributes to reducing the short-term risks associated with hypoglycemia, such as discomfort, dizziness or even life-threatening situations. Diabeloop’s technology, based on artificial intelligence, allows an optimal diabetes management with less glycemic variations responsible for complications, and a life without interruption associated with the chronic disease.
“It is a great satisfaction to see that these new data extracted from an even larger cohort of patients show a simultaneous performance on TIR and reduction of hypoglycemia. Combined with the positive and very concrete feedback from the people equipped, this motivates us to go even further, to address more and more populations affected by diabetes†states Erik Huneker, CEO and co-founder of Diabeloop.
Testimonials: positive impacts in diabetes management, quality of life and mental burden
Since the commercial launch of its first solution in Europe last year, Diabeloop has received numerous feedback on the improvement of diabetes management and clinical benefits.
“I was skeptical about letting my diabetes be controlled by technology. But my diabetologist advised me to try it. My HbA1c dropped from 10% to 7% within two months. DBLG1 has improved my quality of life. I sleep better, I’m fitter, I’m very curious about what the next updates of DBLG1 will bring.†says Martin, living with diabetes for 45 years, equipped in Germany.
“My life has definitely changed with DBLG1. I have much less mental burden. I really noticed that I can have more time for other things and don’t have to concentrate on my diabetes all the time, as long as I take everything responsibly and estimate the carbs of my meals correctly. When I go to parties or some birthdays or other events, I know that DBLG1 always corrects me automatically. I’m really happy!†comments Eva, T1D using DBLG1 in Germany.
“DBLG1 has a “high-fat meal†feature that I use very often, even though I don’t eat that much fat but I don’t eat a lot of carbs either. It allows the delivery of biphasic boluses, adapted to the blood sugar level before meals and it works magically on me to avoid postprandial hyperglycemia!†says Stéphanie, equipped with DBLG1 in France.
 Listen to all the testimonials in the first episode of the exclusive podcast Diabeloop Stories on Spotify : https://open.spotify.com/show/3GNcl…
Listen to all the testimonials in the first episode of the exclusive podcast Diabeloop Stories on Spotify : https://open.spotify.com/show/3GNcl…
Come and meet the Diabeloop team at ATTD, booth n°34 in the TechFair area.
CEO Erik Huneker and Pr Pierre-Yves Benhamou will present these results at Diabeloop session “AID pipeline perspectives & clinical outcomes†on Thursday, 28th April from 12:10 in the Exhibition Area.
About Diabeloop
Created in 2015, Diabeloop is a high-growth company that offers AI-based, personalized solutions to improve clinical outcomes for people with diabetes while relieving them of their constant mental burden. DBLG1 System, Diabeloop’s first medical device for automated insulin delivery (AID) and DBL-hu, its solution for highly unstable Type 1 diabetes management, are both CE-marked and being deployed in Europe.
A second round of financing of 31 million euros, concluded at the end of 2019, supported the international commercial rollout of DBLG1 iController and Diabeloop’s ambitious R&D program. Today, Diabeloop gathers the personality, passion, and skills of over 160 talented individuals who work hard to improve the quality of life for every person living with diabetes.
——————
References:
1 Real-life internal data (YourLoops data extract, from September 1, 2021, to December 31, 2021): 974 patients from Germany, who gave consent, equipped with DBLG1 System with Accu-Chek® Insight (Roche) insulin pump, with HbA1c available at initiation.
2 P.Y. Benhamou et al., «Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial», The Lancet Digital Health, Vol 1 (1), E17 – E25, 2019. DOI: 10.1016/S2589-7500(19)30003-2
3 C. Amadou et al., «Diabeloop DBLG1 Closed-loop system enables patients with Type 1 diabetes to significantly improve their glycemic control in real-life situations without serious adverse events», Diabetes Care 2021 Jan; dc201809. doi:org/10.2337-dc20-1809
4 Data from 152 patients equipped with DBLG1 System with Accu-Chek® Insight (Roche) insulin pump in Germany from April to September 2021 with available HbA1c at initiation, based on [Beck and al. 2019] equation for hb1Ac <=9% and hb1Ac>=6%â€
5 Real-life internal data (YourLoops data extract, from September 1, 2021, to December 31, 2021): 1914 patients from Germany, who gave consent, equipped with DBLG1 System with Accu-Chek® Insight (Roche) insulin pump..
SOURCE: EuropaWire