Myeloid Therapeutics rebrands and unveils an expanded strategy to move beyond its previous myeloid cell focus
- Proof-of-mechanism: Paired biopsies confirmed CAR+ immune cells infiltrating tumors, with immune remodeling and CD8 T cell recruitment.
- Safety and repeat dosing: Over 200 doses delivered with a consistent, manageable safety profile and no cumulative toxicities.
- Evidence of activity: CAR expression detected in circulating immune cells, with stable disease in several patients and a confirmed partial response on treatment for 16 months.
- MT-302 (TROP2; solid tumors): Dose escalation completed; tolerable safety profile.
- MT-303 (GPC3; hepatocellular carcinoma): Dose escalation ongoing.
- MT-304 (HER2; solid tumors): First patient expected Q4 2025; first-in-class multi-immune CAR engaging NK and myeloid cells.
- Retrotransposon-based in vivo CAR-T: First all-RNA product candidate with permanent CAR integration for B-cell depletion.
- Additional multi-lineage programs in preclinical development across oncology and immunology.
- Selective activation: Cell-specific receptors and LNPs for precise T, myeloid, and NK cell programming.
- Flexible durability: Transient or stable CAR expression, with permanent integration via RNA-based retrotransposon technology.
- Industry-leading RNA: Up to 8+ days of linear mRNA expression with no reactogenicity.
- Speed and scalability: Concept-to-clinic in <12 months with low-cost manufacturing.
EU Consortium Launches AI‑Driven “Digital Twin” Platform to Transform Aortic Surgery
 ITALY, 15-Jul-2025 — /EuropaWire/ — An EU‑funded consortium of Italian and French partners has unveiled PANDORA, a pioneering in‑silico platform designed to transform aortic aneurysm surgery, according to a recent press release announcement made on EuropaWire. Developed by LivGemini, RBF Morph, INSA and Université de Rennes (UnivREN) in collaboration with Rennes University Hospital, PANDORA creates patient‑specific “digital twins” of the aorta by combining AI‑driven image analysis, mesh‑morphing algorithms and high‑performance simulations.
ITALY, 15-Jul-2025 — /EuropaWire/ — An EU‑funded consortium of Italian and French partners has unveiled PANDORA, a pioneering in‑silico platform designed to transform aortic aneurysm surgery, according to a recent press release announcement made on EuropaWire. Developed by LivGemini, RBF Morph, INSA and Université de Rennes (UnivREN) in collaboration with Rennes University Hospital, PANDORA creates patient‑specific “digital twins” of the aorta by combining AI‑driven image analysis, mesh‑morphing algorithms and high‑performance simulations.
Surgeons can run virtual procedures on these personalized models to evaluate potential complications—such as graft kinking, impaired coronary perfusion and abnormal wall stresses—before entering the operating room. The goal is to guide prosthesis selection and surgical strategy with objective, data‑backed insights rather than relying solely on individual experience.
Offered under a B2B2B licensing model, the software will be integrated by medical‑device manufacturers into their planning systems for hospitals and clinics. Project coordinator Dr. Leonardo Geronzi, a Forbes “Top Under 30” in Science & Health, says PANDORA aims to shorten operating times, improve postoperative outcomes and reduce costs. After clinical validation at Rennes, LivGemini plans to industrialize the solution for broader market release later this year.
SOURCE: EuropaWire
Sober.Buzz Unveils Three Groundbreaking Initiatives to Strengthen Global Recovery Community

AUSTIN, TX, Unites States, 20-Jun-2025 — /EuropaWire/ — Sober.Buzz, the rapidly expanding online network founded by entrepreneur, investor, and recovery advocate Josh Case, today announces the launch of three key projects designed to deepen connections, amplify hope, and provide tangible support for individuals and their loved ones on the path to sobriety, according to a press release published on EuropaWire.
Building on the vibrant community that congregates around the @SoberBuzzToken Instagram account, Sober.Buzz will roll out:
- “Spreading the Good BUZZ” Podcast
Launching July 7, 2025, Josh Case—celebrating one year plus a day since his “Sobriety Independence Day”—will host weekly, unfiltered conversations with celebrities, entrepreneurs, and addiction experts to share real-life recovery stories and insights. - Online Store & Exclusive Inspirational Collection
Now live, the Sober.Buzz store features custom merchandise celebrating sobriety. The centerpiece: an art-and-fashion line by Heidi Case, designed to inspire and uplift. - Public Sale of the $BUZZ Token
Opening October 1, 2025, the Ethereum-based utility token will fund sobriety-focused philanthropy, unlock premium recovery content, and grant holders access to virtual and in-person community events.

“These launches give our community fresh ways to connect, contribute, and celebrate recovery,” said Josh Case, Founder & CEO. “Our goal is to spread hope, foster engagement, and provide tangible tools for everyone on this journey.”
About Sober.Buzz
Sober.Buzz is a worldwide platform combining social media, digital content, and blockchain to support those affected by addiction. Through storytelling, innovative products, and charitable initiatives, it builds a sustainable ecosystem of hope and recovery.
Photos:


SOURCE: EuropaWire
9th Annual Patients as Partners® Europe Event to Focus on Advancing Patient Involvement in Clinical Research

NEW YORK, NY, United States, 11-Feb-2025 — /EuropaWire/ — The Conference Forum has announced the 9th annual Patients as Partners® Europe event, set for May 20-21, 2025, at the Royal National Hotel in London. This event provides a unique platform for pharma R&D professionals and patient advocates to discuss how patient involvement can reduce risks and inefficiencies in clinical research while advancing the development of new treatments.
Alfred Samuels, a patient advocate and co-chair for 2025, emphasized the importance of recognizing patients as experts in their conditions, which leads to more successful trials and better healthcare outcomes.
Keynote speakers include:
- Harry Verbunt, who will share his post-cancer clinical trial experience and suggestions for improving trial access.
- Peter DiBiaso, discussing the importance of placing patients at the center of research.
- Dr. Temi Olonisakin, offering patient perspectives on support in clinical research.
- Lea-Isabelle Proulx, PhD (Roche), on reducing patient burden through improved protocols.
- Beyza Klein (Johnson & Johnson), on embedding a patient engagement strategy.
- Dr. Catherine Coulouvrat (Sanofi), discussing how patient experience data informs product tolerability.
- Marisa Minetti (Chiesi Farmaceutici), on leveraging patient archetypes in protocol development.
- Gisela Linthorst (Azafaros), on involving the patient community in early clinical trials.
Pharma companies such as AstraZeneca, Boehringer-Ingelheim, GSK, and others will also present case studies on advancing patient partnerships.
The event will explore critical topics including patient-informed research, decentralized trials, increasing trial representation, and reducing patient burden through technology.
Patients as Partners® Europe is co-produced with patients, R&D, academia, and non-profit organizations to advance patient involvement and access. For more details, visit PatientsAsPartnersEU.com.
About the Conference Forum: The Conference Forum is a life science industry leader in organizing conferences, webinars, and digital content, driving efficient medicine development and patient access. Learn more at theconferenceforum.org.
SOURCE: EuropaWire
A Transformative Journey: A Patient’s Experience with Dr. Papaioannou’s Expertise in Facial Rejuvenation Surgery
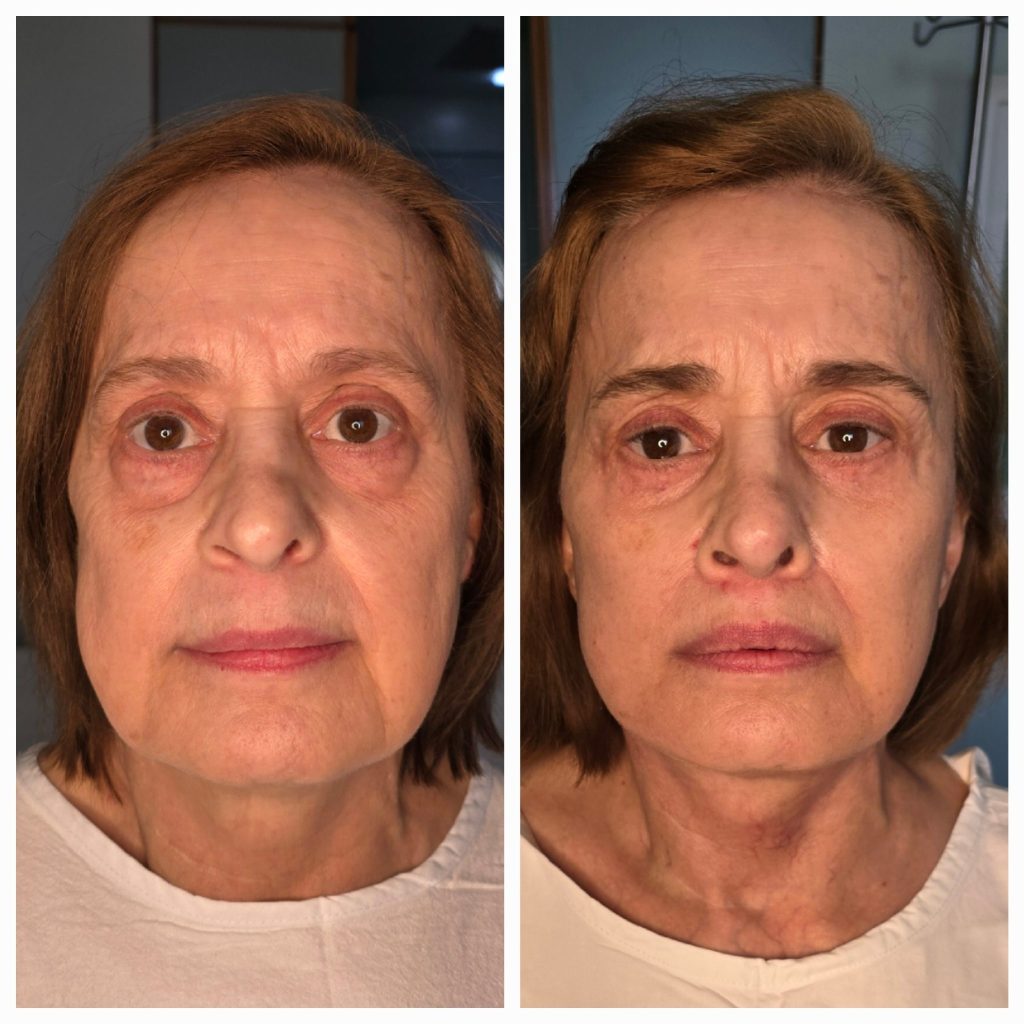
THESSALONIKI, 10-Dec-2024 — /EuropaWire/ — Facial rejuvenation is often seen as a way to regain youthful vitality, but for one patient, it was more than just a cosmetic procedure—it was a journey of self-discovery and renewal. After years of stress, sleepless nights, and the effects of gravity, this patient felt that their appearance no longer reflected how they felt inside. Seeking a solution, they turned to a highly recommended but lesser-known surgeon, Dr. Papaioannou, based in Thessaloniki, Greece.
This decision to travel from the US to Greece for a deep plane facelift was not taken lightly. The patient had heard whispers of Dr. Papaioannou’s artistry, a surgeon known for his precision and attention to detail, but not one who sought fame or attention. Intrigued, the patient researched the surgeon extensively, finding glowing reviews, impressive before-and-after photos, and credentials that promised a high level of expertise.
When the patient finally arrived at Dr. Papaioannou’s clinic, they were immediately reassured by the serene atmosphere and warm, welcoming staff. The surgeon himself exuded a quiet competence, taking the time to listen to their concerns and hopes without rushing or overselling. The plan was clear and tailored to their unique needs—an approach that set the tone for the entire experience.
The surgery itself was smooth and efficient, carried out with care by an experienced team. The patient woke up feeling hopeful, excited to see the results. The transformation was remarkable—beyond just smoothing out wrinkles or tightening skin. Dr. Papaioannou’s skill was evident in the way he had restored a youthful luminosity without changing the essence of the patient’s appearance. The deep plane facelift, combined with a brow lift, eye bag removal, and lip lift, achieved a harmonious balance of facial proportions, revitalizing the patient’s spirit as much as their face.
The recovery process exceeded expectations. Unlike many facial surgeries, there were no complex bandages or compression garments to manage, and the ability to move freely from day one was a pleasant surprise. Swelling gradually subsided, revealing the delicate artistry beneath. The patient’s friends and family noticed a change, sensing a newfound energy and radiance.
Several months post-surgery, the results continue to improve. While the final transformation will take up to a year, the patient already sees a reflection of the vibrant, youthful self they feel inside. Confidence and self-esteem have soared, and the patient now feels rejuvenated and ready to embrace life with a renewed sense of self.
More than a skilled surgeon, Dr. Papaioannou is described as an artist who understands the deep connection between appearance and inner well-being. His expertise has not only transformed the patient’s face but has also rejuvenated their life. With immense gratitude, the patient considers Dr. Papaioannou a hidden gem in the world of plastic surgery, urging others to trust their instincts and embark on the transformative journey that he can offer.
SOURCE: EuropaWire
Goodspace Anxiety Aid and Richard Reid Collaborate to Support Men’s Mental Health for International Men’s Day

SYDNEY, NSW, Australia, 2024-Oct-25 — /EPR Network/ — In recognition of International Men’s Day, Goodspace Anxiety Aid has teamed up with celebrated TV personality and mental health advocate Richard Reid to help raise awareness about men’s mental health. Throughout November, Goodspace is offering a 20% discount on its unique anxiety-relief gum to help support those experiencing stress and mild anxiety. The promotion is available online at www.goodspacegum.com using code MEN20.
Goodspace Anxiety Aid is formulated as a fast-acting, chewable gum that combines five natural ingredients known for their stress-relief benefits, including KSM-66 Ashwagandha, Green Tea, and Passionflower. Engineered to release active ingredients gradually through the gums and cheeks, Goodspace delivers calming effects quickly and conveniently.
Richard Reid, known for his TV appearances on Today and Studio 10 and as an advocate for mental health, is using his voice to help break the stigma around men’s mental health. Reid has spoken openly about his personal struggles and the impact of mental health on his family, and he continues to encourage others to discuss their own experiences.
“Men often face the pressures of mental health in silence,” Reid said. “Partnering with Goodspace Anxiety Aid is a way to help guys feel supported, knowing there are simple, effective options for everyday stress relief. I hope it starts conversations that matter.”
Goodspace gum is widely available at over 1,000 locations across Australia, including Coles and WH Smith. The gum is a convenient option for those looking to manage anxiety on the go, designed to provide fast and effective relief without the need for water or pills.
A spokesperson for Goodspace added, “We’re thrilled to partner with Richard Reid for this campaign. His dedication to mental health awareness is inspiring, and we believe Goodspace can be a useful resource for men facing daily stress.”
Goodspace Anxiety Aid retails for AUD $9.99 and is intended for adults 18+. The discount code MEN20 is valid until November 30. Always read the label, follow directions, and consult a healthcare provider if symptoms persist.
SOURCE: EPR Network
OMRON Introduceert Nieuwe Bloeddrukmeters met Intelligente AFib-Detectie in Europa



KIOTO, Japan, 21-Okt-2024 — /EuropaWire/ — OMRON Healthcare Co., Ltd. kondigt de lancering aan van drie nieuwe bloeddrukmeters in Europa: M6 Comfort AFib, M7 Intelli IT AFib en X7 Smart AFib, voorzien van de Intellisense AFib-technologie. Deze geavanceerde apparaten detecteren automatisch atriumfibrillatie (AFib) tijdens een reguliere bloeddrukmeting, zonder extra stappen.
Intelligente AFib-detectie
Met het Intellisense AFib-algoritme, dat gebruikmaakt van kunstmatige intelligentie, wordt AFib nauwkeurig opgespoord tijdens elke meting, wat het proces aanzienlijk vereenvoudigt. AFib, een hartritmestoornis die beroertes kan veroorzaken, wordt vroegtijdig gedetecteerd, waardoor preventieve zorg toegankelijker wordt.
Klinische validatie
Het algoritme is klinisch getest met resultaten die een gevoeligheid van 95% en een specificiteit van 98% laten zien. Dit bevestigt de betrouwbaarheid van de technologie.
Beschikbaarheid
De nieuwe bloeddrukmeters zijn sinds september 2024 beschikbaar in heel Europa. Voor meer informatie, bezoek de website van OMRON Healthcare.
Over OMRON Healthcare
OMRON is wereldwijd leider in medische apparaten voor thuiszorg, gericht op het verbeteren van de gezondheid en het voorkomen van hart- en vaatziekten.
SOURCE: EuropaWire
OMRON lance en Europe ses nouveaux tensiomètres avec technologie de détection de la fibrillation auriculaire



KYOTO, Japon, 21-Oct-2024 — /EuropaWire/ — OMRON Healthcare Co., leader mondial des technologies de la santé, annonce le lancement en Europe de ses nouveaux tensiomètres à brassard : M6 Comfort AFib, M7 Intelli IT AFib et X7 Smart AFib, dotés de la technologie Intellisense AFib. Disponibles depuis septembre 2024, ces appareils innovants permettent aux patients hypertendus de détecter automatiquement la fibrillation auriculaire (FA) lors des mesures de tension artérielle à domicile.
Détection simplifiée de la fibrillation auriculaire
Grâce à l’algorithme Intellisense AFib, utilisant l’intelligence artificielle, ces tensiomètres détectent les signes de FA directement lors de la prise de tension, simplifiant ainsi la surveillance régulière. La FA, condition cardiaque grave souvent asymptomatique, augmente le risque d’accidents vasculaires cérébraux. Intellisense AFib identifie cette irrégularité avec une sensibilité de 95 % et une spécificité de 98 %, validées lors de conférences médicales prestigieuses en 2024.
Empowerment des patients
Ces nouveaux modèles offrent aux patients un outil essentiel pour une détection précoce de la FA, permettant des interventions rapides pour prévenir les complications. Lucia Prada, Directrice Marketing Senior d’OMRON Healthcare Europe, a déclaré : « Ces dispositifs allient simplicité et technologie de pointe, contribuant à sauver des vies. »
À propos d’OMRON Healthcare
OMRON Healthcare, engagé dans l’amélioration des soins à domicile, propose des produits innovants pour la gestion des maladies cardiovasculaires et respiratoires. Avec plus de 350 millions d’unités vendues dans 130 pays, OMRON poursuit sa vision « Going for ZERO » en éliminant les maladies cérébrovasculaires grâce à ses solutions de santé préventive.
SOURCE: EuropaWire
OMRON Healthcare lancia in Europa i nuovi misuratori di pressione con tecnologia AI per il rilevamento della fibrillazione atriale



KYOTO, Giappone, 19-Oct-2024 — /EuropaWire/ — OMRON Healthcare Co., Ltd., leader mondiale nella tecnologia medica, ha annunciato l’introduzione in Europa dei nuovi misuratori di pressione M6 Comfort AFib, M7 Intelli IT AFib e X7 Smart AFib, dotati dell’innovativa tecnologia Intellisense AFib. Disponibili da settembre 2024, questi dispositivi all’avanguardia offrono ai pazienti un metodo più semplice ed efficace per monitorare la pressione arteriosa e rilevare la fibrillazione atriale (FA) da casa.
La tecnologia Intellisense AFib, basata su un algoritmo di intelligenza artificiale (AI), consente di individuare possibili segnali di FA durante la misurazione della pressione, riducendo la necessità di ulteriori passaggi rispetto ai modelli precedenti. Questa funzionalità è particolarmente importante poiché la FA, spesso asintomatica, è una delle principali cause di ictus, e il monitoraggio regolare consente una diagnosi precoce, fondamentale per prevenire complicazioni più gravi.
I test clinici hanno dimostrato l’efficacia dell’algoritmo Intellisense AFib, con una sensibilità del 95% e una specificità del 98%, convalidando l’affidabilità di questi dispositivi. Questo progresso tecnologico offre ai pazienti ipertesi un potente strumento per monitorare la loro salute, riducendo il rischio di gravi patologie cardiache.
Lucia Prada, direttrice marketing senior di OMRON Healthcare Europe, ha commentato: “I nostri nuovi misuratori di pressione rappresentano un grande passo avanti nella sanità preventiva, grazie alla tecnologia AI che può fare davvero la differenza. Siamo entusiasti di offrire ai pazienti strumenti così innovativi per gestire la loro salute in modo più efficace.”
I nuovi dispositivi sono già disponibili in Europa, e per ulteriori dettagli su prodotti e punti vendita, è possibile visitare il sito ufficiale di OMRON.
OMRON Healthcare si impegna da sempre a sviluppare soluzioni avanzate per il benessere delle persone, contribuendo a ridurre i rischi di malattie cardiovascolari in tutto il mondo. Con oltre 350 milioni di dispositivi venduti a livello globale, l’azienda si conferma leader nel settore della sanità domiciliare.
SOURCE: EuropaWire
OMRON Revoluciona el Cuidado de la Salud en Europa con Tensiómetros Inteligentes Equipados con IA



KIOTO, Japón, 18-Oct-2024 — /EuropaWire/ — OMRON Healthcare ha lanzado en Europa los nuevos tensiómetros M6 Comfort AFib, M7 Intelli IT AFib y X7 Smart AFib, que incorporan la tecnología Intellisense AFib. Estos dispositivos detectan automáticamente la fibrilación auricular (FA) durante cada medición de presión arterial, simplificando el control de esta afección en el hogar.
La FA, una de las principales causas de accidente cerebrovascular, afecta a muchos pacientes sin síntomas evidentes. Con la tecnología Intellisense AFib, basada en inteligencia artificial, OMRON ofrece una herramienta precisa para identificar irregularidades en el ritmo cardíaco y prevenir complicaciones graves.
SOURCE: EuropaWire
Alpha Lifecare Partners with EchoCare to Introduce Cutting-Edge Remote Monitoring Solution for Elderly Care in Australia and New Zealand
Crestview, FL, 2024-May-15 — /EPR HEALTHCARE NEWS/ — EchoCare, a pioneer in remote monitoring solutions for eldercare and senior living, has appointed Alpha Lifecare as the exclusive distributor for its solution in Australia and New Zealand.
“EchoCare is delighted to be working with Alpha Lifecare as our exclusive distributor in the region to ensure that, as the use of our senior monitoring solution continues to expand, customers in Australia and New Zealand will be able to provide better and more efficient care to their clients. As Alpha Lifecare has one of the strongest footholds in the region for delivering world-class innovative technology to senior living providers to improve resident safety and experience, we look forward to building lasting relationships with customers in the region,” emphasized Rafi Zack, COO and acting CTO at EchoCare.
“We are sure that our partnership with EchoCare will enrich our solutions offerings to our customers, as the Digital Angel Radar, developed by EchoCare, offers the most accurate, non-wearable, monitoring solution available, facilitating early detection, targeted intervention, and effective prevention. The system is uniquely capable of monitoring both behaviours and vitals, without privacy-invading cameras and without requiring any action on the part of the older person. We are pleased to have deployed in a first customer site and look forward to rolling out the solution to many more,” said Luke Craddock, Director at Alpha Lifecare.
Explore further information on the Digital Angel Radar solution: https://alpha.global/fallpreventiondetection/
About EchoCare
EchoCare empowers senior living and care providers to enhance their service with around the clock monitoring for improved outcomes. The system learns the senior’s daily activities, facilitating recognition of changes in behaviour – both physical and cognitive – that might indicate increased risk of falls, or other abnormal situations. It also detects emergencies such as falls, wandering, or respiratory distress, which require an immediate response. The company operates globally, with a focus on the US and European markets.
https://echocare.ai/
About Alpha Lifecare
Alpha Lifecare has been delivering and defining solutions for senior care for over 40 years. Their mission is: We make lives better by caring further – helping more people, more often. The company’s technology division aims to deliver world-class innovative technology which improves resident safety and experience whilst also supporting facility teams in enhancing operational efficiencies.
https://alpha.global/
SOURCE: EPR Network
Pharma R&D and patient advocacy to discuss patient involvement in clinical research at Patients as Partners® Europe 2024
NEW YORK, NY, United States, 12-Mar-2024 — /EPR HEALTHCARE NEWS/ — The Conference Forum today announced the launch of the 8th annual Patients as Partners® Europe meeting, taking place May 14-15, 2024, at Plaisterers’ Hall, in London, England. Patients as Partners® Europe offers an unparalleled opportunity to hear from pharma R&D and patient advocacy on how patient involvement gets done to drive greater efficiencies and inclusivity in clinical research with better outcomes.
“Patients as Partners Europe brings advocates and sponsors together in a unique forum focused on improving collaborations that result in meaningful impact for patients,†said 2024 co-chair, Victoria DiBiaso, MPH, Global Head, Patient Informed Development & Health Value Translation, Sanofi.
The 2024 keynotes and featured speakers include:
- Patient Advocate Keynote Derek Stewart, OBE on understanding mental health support in clinical research
- Patient Advocate Keynote Sarah Zenner Dolan, a former biotech executive on navigating clinical trials as a patient
- GSK’s Andrew Garvey, Global Patient Advocacy Lead, on what has been working, and what has not, in GSK’s effort to advance patient engagement in clinical trials
- Pfizer’s Patrick Gallogly, Medical Advisor, Pfizer, on the learnings generated from an industry-first LGBTQ+ oncology advisory board
- Lundbeck’s Anders Lassen, Senior Director, Patient Insights, on using patient-centric integrated evidence approaches to inform drug development decisions
- Boehringer Ingelheim’s Annie Gilbert, Global Patient Advocacy Lead, on the pilot program bridging the communication gap by sending trial updates to patients
- Ipsen’s Oleksandr Gorbenko,​​​​​​​ Global Patient Affairs Director, Neurosciences, on how Ipsen partnered with patient advocacy to create robust patient experience mapping
- AstraZeneca’s Lisa Kerr, Senior Director, R&D Patient Science, on creating a measurement strategy and business investment case to scale patient-centric R&D approaches
- Astellas’ Stephen Head, Senior Director, Patient Partnerships, on instilling conscious awareness of the patient into everyday work practices
- Prostate Cancer Research’s David James, Director of Patient Projects, on demystifying and diversifying clinical trials to engage underserved communities
“Patients as Partners® Europe presents case examples on how patient involvement in clinical research can accelerate medicine development, how it can improve better access, inclusivity and diversity,†said Valerie Bowling, Executive Director.
Key topics to be addressed include:
- ​​​​The future of patient-informed research
- Understanding patient preferences in decentralized clinical trials
- Advancing health equity and diversifying clinical trials to engage underserved communities
- The patient experience data landscape and returning patient data
- Turning patient insights into action
- Mapping and measuring patient engagement
- Patient burden-reducing solutions and technologies
- Regulatory requirements and patient engagement in drug development
The 2024 meeting is co-chaired by ​​​​​​​Sanofi’s Victoria DiBiaso, MPH, Global Head, Patient Informed Development & Health Value Translation; Patient Advocate, ​​​​​​​​​​​​​​Alfred Samuels; and Parexel’s Rosamund Round, VP, Patient Engagement.
About Patients as Partners® Europe:
Patients as Partners® Europe is co-produced with patients, industry, academia, government and nonprofit organizations to establish a well-rounded program that addresses the needs of all stakeholders seeking to implement and advance patient involvement, access and diversity across the entire clinical development continuum. To learn more about Patients as Partners and access the full agenda, visit PatientsAsPartnersEU.com.
About the Conference Forum:
The Conference Forum is a life science industry research, conference development and marketing firm. The company brings the full spectrum of executives together to share ideas and information on how to advance efficient medicine development and delivery, patient diversity and access. They produce trusted conferences, webinars, podcasts, digital editorial and provide marketing services. To learn more, visit theconferenceforum.org.
SOURCE: EuropaWire
IQ Biozoom, a medtech startup, develops non-invasive home diagnostics to measure biomarker levels in saliva with unmatched accuracy
WARSAW, 13-Nov-2023 — /EPR HEALTHCARE NEWS/ — IQ Biozoom, a medical technology startup focusing on developing non-invasive home diagnostics, is set to make non-invasive home testing more convenient with their innovative technology that allows for the possibility to measure the concentration of biochemical substances in liquid analytes such as saliva, sweat, urine or tears. More people around the world will be empowered to monitor the course of various diseases from the comfort of their own home with laboratory precision using saliva analysis. Currently,  non-invasive home tests measuring glucose and lactate levels in saliva and, in the near future, hormones or CRP proteins, are being developed as part of the technology development.
IQ Biozoom’s technology includes a sophisticated biosensor system that integrates inkjet printing technology with advanced semiconductors, specifically thin-film transistors. Although not yet in commercial use, it allows the concentration of a selected biomarker to be determined based on contact between a disposable test strip and a body fluid such as saliva. According to analyses, when measuring glucose concentration in saliva, IQ Biozoom’s cutting-edge technology is up to ten times more accurate than current market standards. This means that even very low concentrations of selected biomarkers in the analyte can be determined with laboratory precision. This can be particularly relevant in those cases where the physiological concentration of a selected substance is very low, or where the mere presence of a biomarker in an analyte is indicative of a health disorder.
Most current blood glucose meters need a finger prick which is usually uncomfortable for patients. IQ Biozoom’s technology enables biomarker levels to be tested with laboratory precision based on saliva analysis, making it a completely non-invasive and painless method.
The solution will enable people with, for example, metabolic and endocrine disorders, to monitor their health and manage their disease, as well as take appropriate preventive care. The devices being developed based on IQ Biozoom’s technology are intended to be used for self-contained, non-invasive home diagnostics, allowing for precise laboratory-standard results.
Monitoring biomarkers and analysing their data retrospectively can help to select the right therapy for a patient. By monitoring biomarkers before, during and after therapy, it is possible to assess whether a patient is responding to treatment. If biomarker levels improve, this may indicate that the therapies are working. If there is no improvement, the therapy may need to be changed. Retrospective analysis of biomarker data can help to personalise therapy, tailoring it to the individual patient. The technology also has applications in personalised sports medicine, particularly in the context of physical performance assessment, training planning and training unit selection. Different athletes may have different lactate threshold which means their training plans should be tailored to their individual body characteristics. Testing lactate levels allows for precise individualisation of training.
Currently, invasive glucometers are the devices of choice for glucose level monitoring. According to the Polish Family with Diabetes report, published by the Association for Diabetes Education SED – 84% of people with type 1 diabetes would prefer to use non-invasive glucose monitoring methods, while 60% skip part of their daily testing due to the need for fingertip pricks. In addition to the discomfort felt, the disruption of the skin increases the risk of an infection. In 2021, IQ Biozoom started developing their innovative technology to non-invasively monitor glucose and lactate levels. Other biomarkers such as cortisol or CRP proteins in the body are in the R&D pipeline.
In the third quarter of 2023, IQ Biozoom obtained funding from the VC Link fund, which supports Polish companies developing breakthrough technologies with global potential. But the seed round is not closed yet with the startup seeking further investment to implement the technology and go to market.
The company expects an even faster growth rate in the near future. The home diagnostics market is growing at 5.6% year-to-year and is expected to exceed $13.6 million by 2033, according to FutureMarketInsight.com.
About IQ BIOZOOM
IQ BIOZOOM Ltd (https://iqbiozoom.com/) develops technology for non-invasive home diagnostics. The company is working on innovative devices for measuring the concentration of biochemical substances in saliva: glucose, lactates, and hormones, to enable people with metabolic and endocrine disorders to monitor their health and manage their disease, as well as applying appropriate prevention. IQ Biozoom technology will be designed for home self-testing. In the near future, the company intends to focus on expanding the technology usage to measure the levels of other substances important for diabetics (diabetes package) and woman (hormone package) ‒ in saliva.
SOURCE: EuropaWire
A groundbreaking paper: Climate change is a key driver of infectious disease outbreaks in Europe

BRUSSELS, 5-Sep-2023 — /EPR HEALTHCARE NEWS/ — The Lancet Regional Health – Europe journal has published a scientific paper on 7 August 2023 authored by a distinguished team of international researchers as part of their work in the IDAlert project. Titled “Decision-Support Tools to Build Climate Resilience Against Emerging Infectious Diseases in Europe and Beyond“, this paper introduces a transformative approach to tackle the emergence and transmission of climate-sensitive infectious diseases in Europe, informing cross-sectoral policy while improving the long-term climate resilience of health systems to infectious disease risks.
Climate change is one of several drivers of recurrent outbreaks and geographical range expansion of infectious diseases in Europe. This paper proposes a collaborative approach to develop policy-relevant indicators and decision-support tools. These tools are designed to comprehensively track and anticipate climate-induced disease risks across various domains, including environmental hazard, exposure patterns, and vulnerability factors. With a keen focus on the interconnectedness of animals, humans, and the environment, the framework promises a holistic perspective to address this multifaceted challenge.
The lead author and IDAlert project coordinator Joacim Rocklöv highlighted, “Our decision-support tools offer a multi-dimensional perspective that transcends traditional silos. By examining the nexus of animals, humans, and the environment, we’re unlocking a more comprehensive understanding of disease dynamics a pre-requisite for more timely and effective outbreak preparedness.â€
The heart of this novel framework lies in the co-production of early warning and response systems with stakeholders and end-users, as well as tailored tools to assess the costs and benefits associated with climate adaptation and mitigation strategies across diverse sectors. By fostering greater resilience within regional and local health systems, the framework aims to strengthen Europe’s capacity to respond to health crises, even in the face of changing environmental conditions.
As part of its approach, the IDAlert project will integrate multi-level engagement, innovative methodologies, and novel data streams, and tap into locally generated intelligence and empirical insights through case studies. This strategy empowers experts to quantify the effects of climate-induced disease threats in areas undergoing rapid urban transformation and contending with heterogeneous health risks. The ultimate aspiration is to bridge the gap between knowledge and action, delivering an unparalleled integrated One Health—Climate Risk framework that will empower policymakers, healthcare professionals, and communities to mitigate risks and bolster resilience.
About The Lancet Regional Health – Europe Journal:
The Lancet Regional Health – Europe Journal is a prestigious journal renowned for publishing groundbreaking research on health challenges worldwide. It aims to promote the advancement of the research agenda, clinical practice and health policy in Europe with the goal of improving health outcomes for all people regionally and globally.
About the IDAlert project:
IDAlert – Infectious Disease decision-support tools and Alert systems to build climate Resilience to emerging health Threats – officially started on 1 June 2022 is a € 9.18 million project and lasts for five years. The project is funded by the European Commission under the Horizon Europe programme with Grant Agreement number 101057554. More information: www.idalertproject.eu
Note to Editors:
For more information about the paper or to request an interview with the researchers, please contact the IDAlert project: contact@idalertproject.eu.
The full paper, “Decision-Support Tools to Build Climate Resilience Against Emerging Infectious Diseases in Europe and Beyond,†is available on the Lancet Regional Health Journal website:
https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(23)00120-5/fulltext
SOURCE: EuropaWire
AMBROSIA, a new HORIZON-RIA project, aims to develop a portable diagnostic unit for intelligent sepsis diagnosis
ATHENS, 9-May-2023 — /EuropaWire/ — AMBROSIA (http://ambrosia-h2022.eu/) is a new ambitious HORIZON Research and Innovation Action project that was granted under the call of “Digital and emerging technologies for competitiveness and fit for the green deal†and the topic “HORIZON-CL4-2022-DIGITAL-EMER-GING-01-03: Advanced multi-sensing systemsâ€. AMBROSIA envisions to develop a portable diagnostic unit for intelligent sepsis diagnosis at the point-of-care (PoC).
Sepsis is a life-threatening whole-body inflammatory reaction caused by a severe infection. With mortality rates around 35%, sepsis is responsible for 11 million deaths worldwide every year. In economic terms, sepsis represents a primary cost of hospitalization in developed regions, expending up to €9 billion/year only in Germany. The window of opportunity for sepsis management is in hours: the chance of survival drops by 7.6% each hour of disease progression until an appropriate treatment is started. However, early and accurate sepsis detection is hampered due to: i) complex diagnostic criteria requiring screening of multiple targets, (ii) time-consuming methods for identifying the bacterial causes of sepsis, (iii) specimen transfers in centralized laboratories. Meeting this challenging framework can only be accomplished through the adoption of real-time and label-free sensor technologies incorporated in PoC platforms.
This is exactly where AMBROSIA steps in to transform integrated plasmo-photonic RI sensors into a disruptive solution for sepsis diagnosis at the point of care that will offer multiplexed (within a single test) quantification of multiple protein biomarkers and bacteria within a few minutes, providing also real-time disease stage classification and enabling a rapid and precise decision making for medical actuation. Specifically, AMBROSIA objectives include:
1. Development of multi-channel label-free CMOS plasmo-photonic sensors with high-sensitivity and resilience to noise.
2. Hetero-integration of InP-on-SiN through micro-transfer printing (μTP).
3. Development of an AI-based electro-optical read-out exploiting ultra-low power photonic Deep Neural Networks for disease classification.
4. Development of a PoC sepsis diagnostic unit.
5. Experimental validation of AMBROSIA sepsis diagnostic system with real samples.
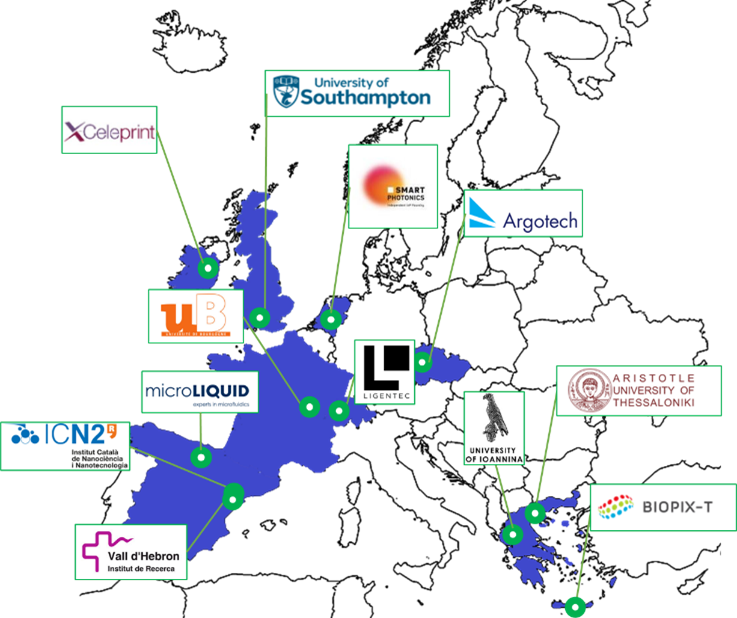
The project is coordinated by the Aristotle University of Thessaloniki, Greece, and is scheduled to run for 4 years bringing together 12 partners strategically compiled from strong industrial and academic organizations including: (a) 4 universities, Aristotle University of Thessaloniki (GR), University of Bourgogne (FR), University of Southampton (UK), and University of Ioannina (GR), (b) 2 research institutes, the Catalan Institute of Nanoscience and Nanotechnology (ES), and the Vall d’Hebron Research Institute (ES), and (c) 6 companies, Ligentec (CH), SMART PHOTONICS BV (NL), X-Celeprint (IE), Argotech (CZ), microLIQUID (ES), and Biopix DNA Technology P.C. (GR).
About AMBROSIA
A MULTIPLEXED PLASMO-PHOTONIC BIOSENSING PLATFORM FOR RAPID AND INTELLIGENT SEPSIS DIAGNOSIS AT THE POINT-OF-CARE
Project ID:101093166
Call: HORIZON-CL4-2022-DIGITAL-EMERGING-01
Type of Action: HORIZON-RIA
Start Date: 01 Jan 2023
Duration: 48 months
Estimated Project Cost: €4,999,612.17
Requested EU Contribution: €3,689,509.66
Website: http://ambrosia-h2022.eu/
About BIOPIX-T
BIOPIX DNA TECHNOLOGY P.C. (https://biopix-t.com)  was founded in December 2019 in order to commercialize a novel molecular diagnostic method, developed by the founding team, for the detection of nucleic acids at the point-of-care. The company targets multiple healthcare-diagnostics related markets through the production of standardized assays for detection and monitoring of Infectious Diseases and Companion Diagnostics. Our mission is to offer portable diagnostic devices to every potential end-user, regardless of financial status, geographical location and training.
SOURCE: EuropaWire
Novartis, Lundbeck, and GSK Among Pharmaceutical Companies to Present at Patients as Partners Europe 2023
NEW YORK, NY, United States, 13-Apr-2023 — /EPR HEALTHCARE NEWS/ — The Conference Forum announced that the 7th annual European Patients as Partners® in Clinical Research conference will return to London at the Thistle Hotel Marble Arch on June 12-13, 2023. The event will showcase the progress made by pharma R&D executives with input from patients on achieving greater representation in clinical research, building trust and engagement in communities, and building truly patient-centric R&D organizations.
“We are delighted to be back in person to offer an unparalleled opportunity to hear from pharma R&D and patient advocacy together on how patient involvement gets done to drive greater efficiencies in clinical research with better outcomes,†said Valerie Bowling, Executive Director, Patients as Partners in Clinical Research Europe.
A variety of pharmaceutical companies will report on the progress they’ve made engaging communities and partnering with patients. Sanofi will open with the evolution of patient-informed research and its impact on medicine development. Novartis will be speaking on the organization’s global approach to implementing systematic and consistent patient engagement across the medicine lifecycle with a focus on measurement.
Lundbeck will share how they are generating insights through engagement with patient communities and making patient input actionable for medicines development. CSL Behring will discuss how they are evaluating clinical research sites based on patient-focused principles.
Several companies, including Regeneron, the Center for BME Health and Sanofi, will discuss their progress in increasing diversity amongst underrepresented communities in clinical research. GSK will lead a session on understanding barriers to community engagement in clinical research.
Boehringer Ingelheim will discuss the company’s approach to engaging patients throughout the clinical trial and how to leverage those patient insights to improve the process. Alnylam Pharmaceuticals will share how the organization has developed a top-down approach to integrating the patient perspective.
The conference features incredible patient advocate representation, addressing the realities of underrepresented communities to better serve them. For the first time, Patients as Partners Europe will have a patient-led session on supporting mental health for patients in clinical research.
“I look forward to hearing from patients and industry colleagues about what matters most and what has the greatest impact when partnering with patients and their communities on our efforts to new therapeutic options available,†said Deirdre BeVard, SVP, R&D Strategic Operations, CSL Behring and Patients as Partners Europe co-chair.
Key topics for 2023 include:
- The evolution of patient informed research
- Increasing diversity in clinical research
- Patient access to research through digital innovation
- Understanding barriers to community engagement in clinical research
- Turning patient insights into action
- Navigating patient engagement and compliance
- Mapping and how to measure patient engagement
- Regulatory requirements and patient engagement in drug development
To learn more about the event, and a full overview of speakers, please click here (https://theconferenceforum.org/conferences/patients-as-partners-europe/overview/)
About Patients as Partners EU
Patients as Partners is co-produced with patients, industry, academia, government and nonprofit organizations to establish a well-rounded program that addresses the needs of all stakeholders seeking to implement and advance patient involvement across the entire clinical development continuum.
About the Conference Forum
The Conference Forum is a life science industry research firm that develops conferences primarily around how to get therapeutics to patients faster. They examine and challenge the complex ecosystem of drug development and delivery, bringing ideas together from a variety of sources to help advance clinical research with common goals that are patient-focused.
They currently offer conferences for pharma/biotech professionals including R&D leaders, CEOs, business development/licensing, medical affairs/safety, chief patient officers/advocates, clinical innovation champions, investors and drug delivery specialists. The company also publishes six newsletters and produces PharmaTalkRadio and virtual events.
SOURCE: EuropaWire
First patient enrolled in an international multicenter clinical study for non-invasive cardiac resynchronization therapy
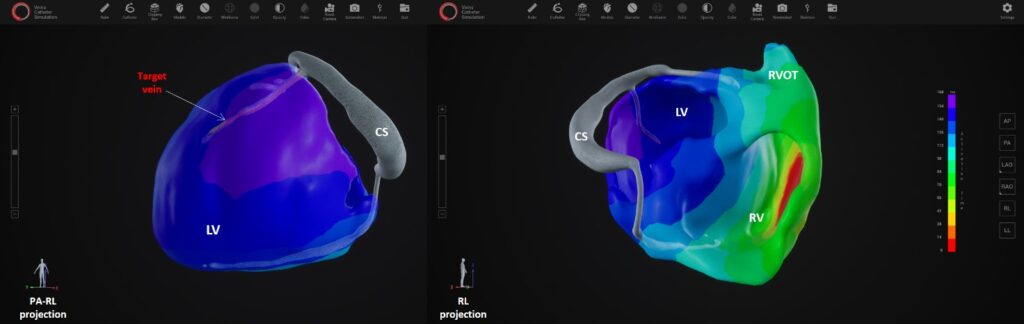
BOLZANO/ BOZEN, Italy, 19-Mar-2023 — /EPR HEALTHCARE NEWS/ — XSpline SPA, a start-up company incorporated in Bolzano (Italy) and the Ordensklinikum Linz Elisabethinen Hospital in Linz (Austria) today announced the first patient enrolled in a clinical study (ClinicalTrials.govID: NCT05327062) for the cardiac resynchronization therapy (CRT) guided by non-invasive electrical and venous anatomy assessment.  The international multicenter prospective study will include 150 patients from 16 centers in the U.S. and Europe.
XSpline Cloud provides a cloud-based non-invasive cardiac panoramic mapping technology to select individual CRT treatment strategy and predict outcomes.
Fast and fully automatic Artificial Intelligence based epi- and endocardial segmentation of cardiac structures, including coronary sinus veins, provides clear understanding of individual patient’s anatomy. The electrical assessment is based on a 3D panoramic electro-anatomical map based on 12 -lead ECG only, without requiring multichannel ECG recording or any kind of body surface potential mapping. XSpline Cloud is the first system in the world with AI based identification of the correct target zone for LV lead implantation.
The cloud-platform guarantees full operability with existing clinical standards and data formats; it supports IHE profiles, HL7 FIHR, DICOM, and a broad range of ECG formats. The platform also provides embedded medical grade visualization tools, such as an integrated multimodality DICOM viewer, an ECG viewer with measurement capabilities, a high-performance 3D viewer for segmented cardiac structures and electro- anatomical maps as well as an interactive navigator for LV lead placement.
Dr. Georgios Kollias, M.D., is the principal investigator for the clinical trial in Austria and performed the first procedure on a 71-year-old patient with ESV LV of 154 ml and EF LV of 20%.
“The XSpline Cloud software provides a unique approach to selecting an individualized CRT treatment strategy. It is a comprehensive tool for successful CRT implantation that can also predict outcomes, making the implantation procedure faster, easier, and safer for the patient. We are very pleased to start using this tool in our clinicâ€, said Dr. Kollias.
“XSpline SPA is supported by an international R&D team of skilled clinicians, mathematicians, and biomedical engineers with the shared goal of improving patient care. We think that the real-time visualization of each patient’s individual anatomy, the precise non-invasive electro-anatomical endo- and epicardial activation map and the interactive navigator will help to increase CRT respondersâ€, said Mr. Werner Rainer, CEO of the company.
XSpline Cloud is an investigational device and not yet approved for commercial use.
SOURCE: EuropaWire
MIGUN LIFE, the original brand of K healthcare, to participate at CES 2023

SEOUL, Korea, 20-Dec-2022 — /EPR HEALTHCARE NEWS/ — MIGUN LIFE (CEO Jacob Hun Cheol Chang), the originator of home medical appliance, will participate in CES 2023 world’s largest exhibition of home appliances which will be held on January at Las Vegas Convention Center in USA, and will unveil year 2023 new products.
MIGUN LIFE set up exhibition booth in Convention Center at Venetian Expo Hall and will introduce MIGUN LIFE’s products and brand to global buyers. The year 2023 new products to be unveiled this time are Re:ach RH01 and Re:ach RH02.
Product Information
Re:ach RH01 is product in a form of mat that allows us to enjoy relaxation and spine treatment. Re:ach RH02 is product in a form of mattress that allows us to enjoy both sleeping and spinal treatment which looks like a regular bed. The main feature of these products is that hidden jade massager comes out and starts spinal massage from one’s neck to hip. More than 10 acupressure rods stimulate blood spots exactly next to spine line. Once the operation is stopped, jade and acupressure rods return to inside of the mat or mattress, then you can have comfortable relaxation and sleep. Each product has leg cuffs exclusively for lower body which has functions for massaging legs and acupressure for soles of the foot. Also, they are evenly equipped with all convenient functions necessary for massage and relaxation such as thermal function, body scanning function, massage function tailored to individual’s body shape, intensity modulation massage function, relaxing music.
Personal thermal therapy appliance which was upgraded through MIGUN LIFE’s unrivaled technology Re:borne, personal ultrasound stimulating appliance Re:vive, high-frequency foot stimulator DAY:RE, these 3 products will be also exhibited.
Exhibition Booth
In the booth, products are placed in relaxed space so that visitors can experience the products comfortably and reception space for business talk are prepared separately. Furthermore, key component which shows MIGUN’s advanced technology, 2 sets of 5 thermo jades (10 thermo jades) will be exhibited with an emphasis on visitors to experience and understand MIGUN LIFE. 2 sets of 5 thermo jades (10 thermo jades) are made of 10 processed natural jades which have thermal function and it moves along the spine line and provides compression and heat.
Purpose of Participation
MIGUN LIFE has purpose to achieve from participating CES 2023. First of all, it is to promote brand and products worldwide to expand our global business in earnest from 2023 which we established 50 countries and for about 30 years. Also, it is to increase sales across the US since the establishment of US corporation in 2000. MIGUN LIFE has export record more than 5 million US dollars in the US, proceeded FDA (Food AND Drug Administration) for approval but also clinical trial at UC Irvine in the US. It explains that if MIGUN LIFE meets demand from American customers, the products will be recognized as differentiated product by going beyond global standards.
An official from MIGUN LIFE said “MIGUN LIFE, the original brand of K-healthcare, will participate CES 2023 to introduce 2023 new products and is asking for interest of MIGUN LIFE’s active role in Korea market but also in global market as wellâ€
Homepage :Â http://www.migunlife.co.kr
Inquiry :Â +82-2-6951-2886 / Sales Rep: Andrew Yoon (migunlife_gsales@migunlife.co.kr)
Sales Rep:Â Andrew Yoon (migunlife_gsales@migunlife.co.kr)
SOURCE: EuropaWire
A British Girl Creates a Special Sign for Women’s Periods to Say “No†to Period Shaming

NEW YORK, 2022-Nov-21 — /EPR HEALTHCARE NEWS/ — In recent years, “menstrual shame†has become one of the most searched topics on the Internet, which caused heated discussion among netizens. Some women said they had never felt ashamed of menstruation and they even could talk about it openly. Some others said they felt period-shame when they were young; however, this feeling gradually disappeared as they grew older…
It is believed that most girls feel embarrassed or even ashamed to talk about periods when they are young. They hesitate to tell others when they feel sick during the periods; they even feel uncomfortable buying sanitary essentials from shops and try to “cover up†the embarrassment with black plastic bags.
Periods shouldn’t be taboo!
Sophia McKinstry, a girl from a university in UK, has exquisitely made a “Special Sign for Women’s Periods†to call on people to break the taboo around menstruation, remove the shame and stigma, and urge the society to face women’s physiological periods squarely. The overall outline of the Special Sign for Women’s Periods is like a drop of blood. The upper part is mainly composed of rosebuds, which shows a curling-up female who is lying on her side. The hollow part showing a few bat totem elements combines with the red shadow below to express that the woman is on her period.
When talking about the chance for the creation of the Special Sign, Sophia McKinstry said that she initially just wanted to help girls with period discomfort by designing a special sign, for example, when others see a girl wearing this sign, they will know that she is on her period and may feel tired and uncomfortable. In some cases, she probably needs a little care and understanding like a hot drink for her when eating meals together or avoiding persuading her to drink wine. She saw a piece of news that Melinda Gates liked the Chinese swimmer Fu Yuanhui for her bravely smashing period taboo during the Rio Olympics and noticed various hot discussions triggered by the Indian film Padman. After that, she gradually realized that period shame is a common phenomenon in many countries. Menstruation is perceived as unclean or shameful by girls and other people, which can be seen in all aspects of our lives. Therefore, she began to pay more attention to the topic of menstrual shame.
Sophia McKinstry stated that she wanted to make people understand through her own way that menstruation is a normal and healthy part of life for women and the traditional idea that periods are dirty should be bravely broken. Female periods should be treated equally as other physical phenomena in the society and they should not be taboo. For this purpose, she “encourages people to abandon the traditional secular ideas such as ‘menstrual taboo’ and ‘ menstrual shame’â€, and integrate “may every girl does not feel embarrassed or ashamed any more when talking about menstruation†as well as other concepts and appeals into the design concept of the Special Sign.
The Special Sign for Women’s Periods has become globally accepted to mark women’s menstruation and periods. It encourages women to break the taboo of “menstrual shameâ€, calls on the society to stop hating “menstruationâ€, and represents care and understanding for women on periods.
The small sign contains infinite power. We believe that it will promote a great spirit, end the long-standing discrimination, and arouse people’s courage to challenge the secular ideas and break taboos, just like the Olympic Rings and Red Ribbons.
Via EPR Network
More Healthcare press releases