PARIS, 2-Jun-2022 — /EPR HEALTHCARE NEWS/ — Diabeloop secures 70 million euros to pursue its solid and sustained growth strategy.
The company, an Automated Insulin Delivery pioneer, develops machine-learning software for diabetes treatment. Its highly sophisticated algorithms are integrated into easy-to-use products helping facilitate chronic disease management impacting patients’ clinical outcomes and their quality of life.
Diabeloop has seen impressive growth in the last few years with two products on the European market, rapidly approaching 10,000 equipped patients one year after commercial launch and major developments with various device partners.
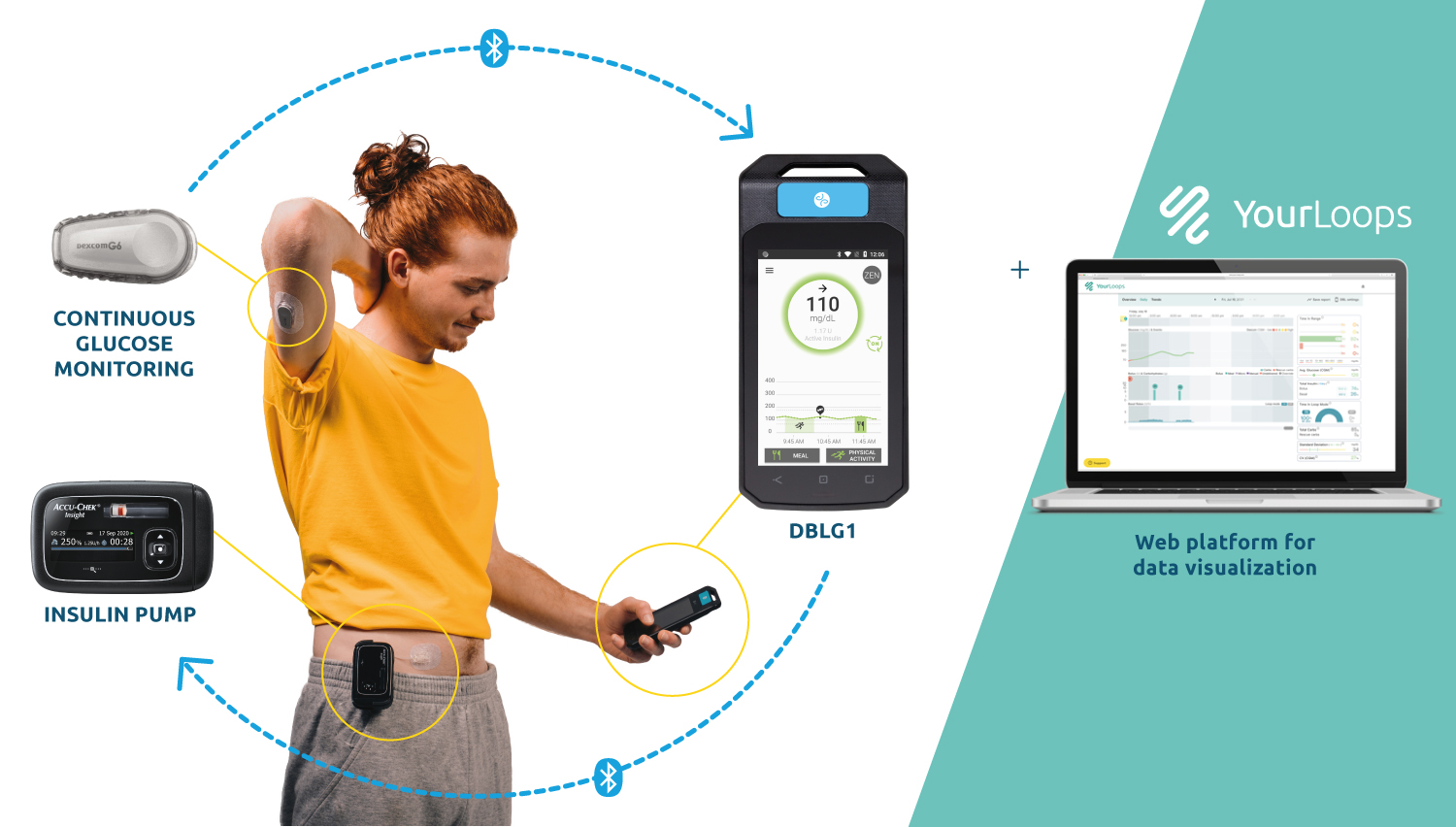
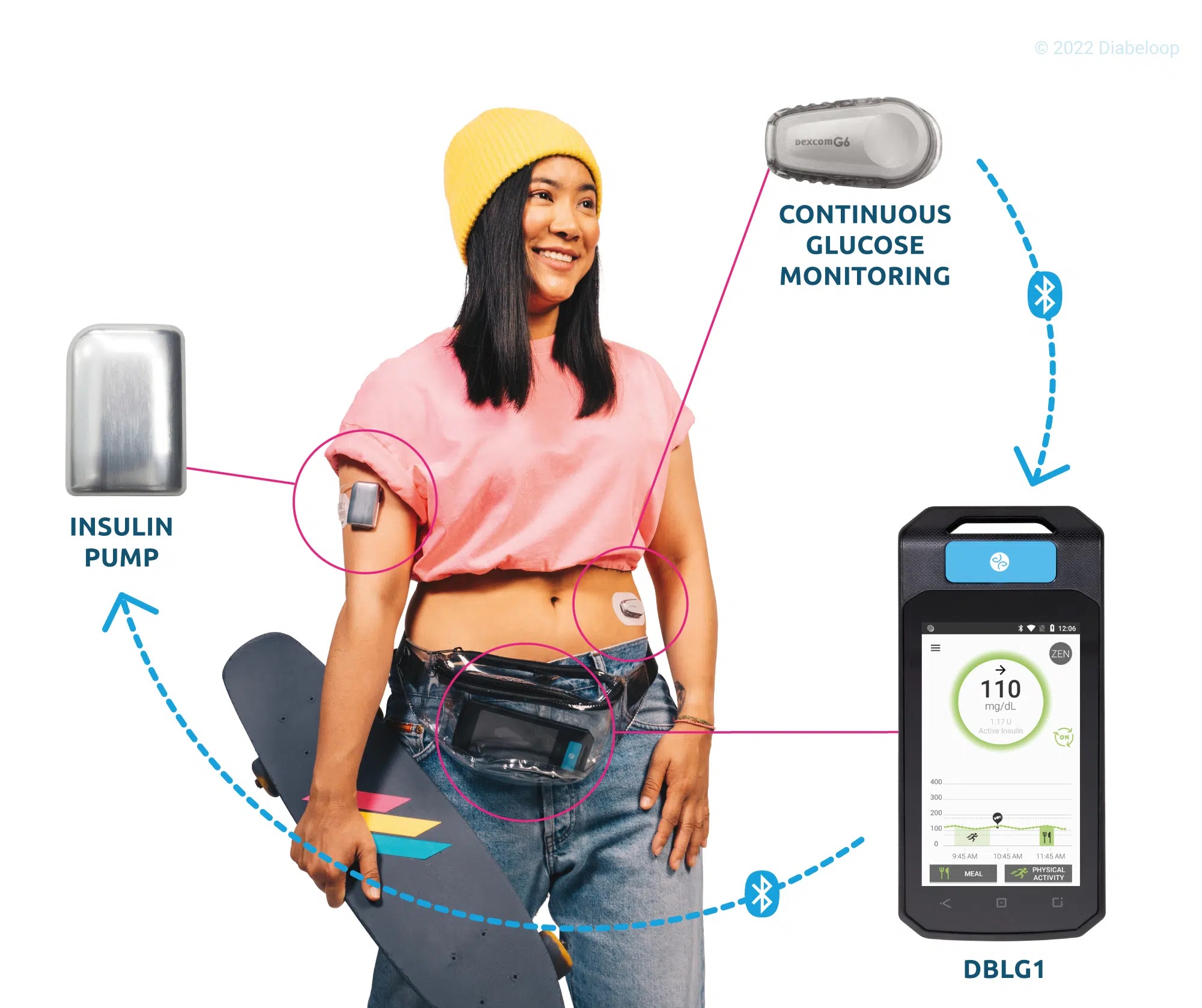
The first closed-loop solutions commercialized by Diabeloop offer personalized management for Type 1 (DBLG1) and highly unstable Type 1 diabetes (DBL-hu). By connecting a Continuous Glucose Monitoring device (CGM) and an insulin pump, Diabeloop’s world-leading algorithms analyze data in real time and automate insulin delivery. It transforms the lives of people with diabetes by taking over many of the therapeutic decisions that used to fall to patients.
Erik Huneker, founder & CEO of Diabeloop, declares: “Being able in 2021 to bring the benefits of our products to thousands of patients and hearing their enthusiasm, is why all of us at Diabeloop work so hard. The financing round announced today recognizes the team’s successes, and more importantly, the great opportunities ahead.â€
The financing round is led by prominent private equity player LBO France. The Venture division of the sustainability-conscious private equity fund is a European leader in digital health, supporting young companies with ambitious projects showing international footprints.
“Diabeloop’s team has developed a unique data driven product that has the potential to revolutionize the daily life of millions of diabetic patients by taking away most of their daily mental burden and significantly improving the control of their disease. We are eager to join the investor base and help the company become a global leader in diabetes closed-loop software solutions,†says Valery Huot, Partner, Head of Venture at LBO France.
International healthcare leader Terumo Corporation also participates in Diabeloop’s Series C round. After entering into development agreements with one another in 2020, Terumo extended this to a comprehensive strategic partnership agreement with Diabeloop in 2021.
“We are excited that Diabeloop successfully completed this important milestone and the great opportunities will continue to expand for all corners of the world. Together with Diabeloop, we are committed that we will be able to contribute to all stakeholders by providing patient-centric and personalized solutions,†comments Hikaru Samejima, President of Terumo’s Medical Care Solutions Company.
Innovacom, a key player in the French and European startup community specialized in deep-tech and industrial projects, joins LBO France and Terumo Corporation as new investors.
Comforted by the commercial launch of Diabeloop first devices and the break-through potential of its projects to address a 10-million patients market in the mid-term, previous investors CERITD, CEMAG invest, Kreaxi, Supernova Invest, AGIR A DOM., Crédit Agricole, Odyssée Venture, UI Investissement (Sofimac) and Promontoires all have decided to reinvest.
Additionally, Bpifrance and the historical bank pool are supporting Diabeloop via debt financing.
“We are grateful for the confidence of our existing investors and proud of our distinguished new financial and industrial shareholders. I have full trust in the talent and commitment of the Diabeloop teams to pursue their groundbreaking value creation path for patients and become a trusted leader and partner in the highly reactive diabetes market,†concludes Catherine Dunand, Diabeloop’s Chairman of the Board.
In 2019, Diabeloop secured the highest recorded European financing round in Therapeutic Artificial Intelligence and went on to commercially launch two products, with several thousand patients equipped in less than a year. Their CE-marked data visualization platform accompanies healthcare professionals and patients as a telemedicine and remote patient monitoring solution. High-impact projects such as the adaptation of the core-algorithm to the multi-million users Connected Pen market and development of smart-watch devices also have contributed to Diabeloop’s tremendous momentum.
About Diabeloop
Diabeloop’s mission: Making innovation accessible to people living with diabetes, improving clinical results while relieving them of their constant mental burden.
Created in 2015, Diabeloop is a high-growth company that offers AI-based, personalized solutions to automate the treatment and the management of diabetes. DBLG1 System, Diabeloop’s first medical device for automated insulin delivery (AID) and DBL-hu, its solution for highly unstable Type 1 diabetes management, are both CE-marked and being deployed in Europe. Diabeloop just completed its Series C financing round, securing 70 million euros, to accelerate its commercial roll-out, support its sustained growth strategy and its high-impact projects.
Today, Diabeloop gathers the personality, passion, and skills of over 160 talented individuals who work hard to improve the quality of life for every person living with diabetes.
About LBO France
With €6.6 billion of capital raised since its inception, as a key player in private equity, LBO France is supporting growing French and Italian companies for more than 30 years.
Its investment strategy is based on five distinct segments managed by dedicated teams:
(i) Mid Cap Buyout through the White Knight funds, and Small Cap Buyout through the Small Caps Opportunities funds,
(ii) Venture capital through the Digital Health funds,
(iii) Real estate through the French White Stone funds, OPCI Lapillus, impact fund Newstone and the Italian management company Polis Fondi SGR
(iv) Debt, notably in Italy through the Vita Superbonus program to finance energy efficiency in real estate assets
(v) Public Equity through the France Développement fund
LBO France is 100 % owned by its management and employs nearly 70 professionals.
SOURCE: EuropaWire