NEW YORK, 21-Mar-2022 — /EPR HEALTHCARE NEWS/ — Every year, over 7 Million people are known to die by suicide, one of the major concerns of mental health, according to the statistics by the World Health Organization (WHO). Calculatedly, this comes to one person every 40 seconds. Moreover, in the year 2019, suicide accounted for 1.3% of the total number of deaths around the globe. On the other hand, mental health disorders, such as depression, affected 3.8% of the global population, or around 280 Million people. This included 5.0% adults and 5.7% aged adults above 60 years of age.
Kenneth Research recently released a report titled “Global Mental Health Software and Devices Market†which includes a detailed analysis of the market dynamics along with the impact of COVID-19 on the market growth during the forecast period, i.e., 2021-2030. The report also includes the regulatory and standard landscape, and in-depth research about the market growth across the five major regions, i.e., North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
In Europe, Austria (24 males, 5.6 females), Belgium (23 males, 8.6 females), France (22 males, 5.9 females), Finland (22.9 males, 7.6 females), Germany (17.3 males, 4.8 females), Sweden (17.7 males, 7.0 females), Netherlands (15.7 males, 7.0 females), has shown the highest number of death rates and self-harms per 100,000 inhabitants.
As COVID19 sweeps the global economies, Europe is one of the worst hit since 2020. As new COVID guidelines, lockdown and restriction comes into force, economies have reported elevated cases of anxiety and stress. Especially quarantine and its ill impacts have pushed levels of loneliness, depression, drug use, alcohol consumption, suicidal behavior and self-harm are pushed to another level.
Request for Research Report Sample-Â https://www.kennethresearch.com/report-details/mental-health-software-and-devices-market/10352350
Mental health is a major concern globally and with the rising prevalence of different types of such disorders in recent years, as well as with the growing awareness for these diseases by governmental bodies, the disease has been included in the list of 17 Sustainable Development Goals (SDGs) of the United Nations. Moreover, the situation for the disease has also gained massive focus, especially to treat the elderly population. This can be attributed primarily to the increasing elderly population count over the past few years, and the growing awareness for mental health and well-being amongst these age groups. In the other statistics by the WHO, the proportion of the population of age 60 years and over around the world is expected to double from 12% to 22% between 2015 and 2050. The statistics further stated that over 20% of these adults suffered from a mental or neurological disorder, and 6.6% of all disabilities amongst these population groups accounted for such disorders. Additionally, around 5% and 7% of these populations had been affected with dementia and depression respectively, which are the two most common mental and neurological disorders.
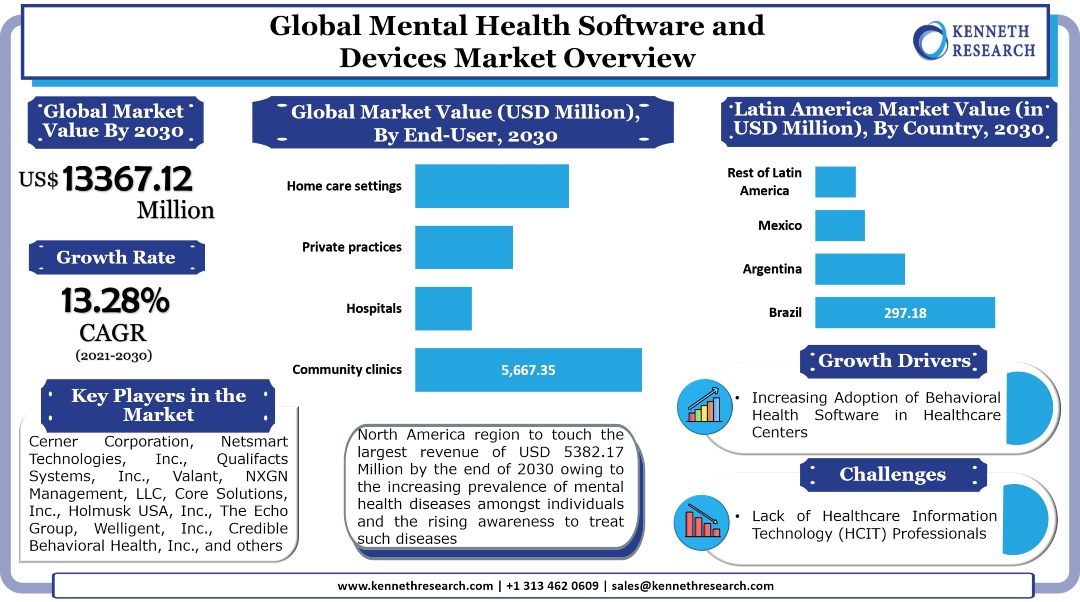
The global mental health software and devices market generated a revenue of USD 3883.94 Million in the year 2020 and is further expected to grow with a CAGR of 13.28% during the forecast period and touch a value of USD 13367.12 Million by the end of 2030. The growth of the market can primarily be attributed to the growing need for advanced patient monitoring and patient management applications amongst healthcare service providers for those treating mental disorders, backed by the surge in the number of mental health patients worldwide. Besides this, the increasing number of these patients is expected to drive the need for advanced devices which can help for early diagnosis and treatment, therefore contributing to the market growth in the coming years. For instance, the prevalence of dementia, which is known for deteriorating the memory, thinking, behavior, and the ability of the patients to perform everyday activities, is expected to increase to 82 Million by 2030, and further touch 152 Million by 2050, according to the statistics by the WHO. On the other hand, according to the statistics by United Nations Children’s Fund (UNICEF), in the year 2019, between the ages 10 and 14, 34,840,000 female adolescents and 44,647,000 male adolescents suffered from mental disorders. Moreover, between the ages 15 and 19, 41,712,000 female adolescents and 44,563,000 male adolescents had suffered from the same disorder. The surge in these cases is expected to boost the demand for diagnostic devices for the treatment of the disorder, and in turn, drive the market growth.
Request for Research Report Sample-Â https://www.kennethresearch.com/report-details/mental-health-software-and-devices-market/10352350
The growth of the global mental health software and devices market can also be attributed to the increasing number of government initiatives that focus on maintaining good mental health and well-being of the individuals of nations worldwide. For instance, in May 2013, during the 66th World Health Assembly, the Comprehensive Mental Health Action Plan 2013-2020 developed by the WHO was adopted by the Ministers of Health of the 194 Member States. This action plan was further extended until 2030 in the year 2019, during the 72nd World Health Assembly. On the other hand, according to the statistical report titled “Mental Health Atlas 2020â€, published by the WHO, 75% of the Member States had a stand-alone policy or plan for mental health. This was an increase from 68% in the year 2014.
Additionally, the statistics also stated that 57% of the Member States had a stand-alone mental health law. This was an increase from 51% in the year 2014. Besides this, since 2017, 46% of the WHO Member States had updated their health policy or plan, while 27% of them had updated their law regarding mental health.
The global mental health software and devices market is segmented on the basis of region into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. Out of the market in these regions, the market in North America generated the largest revenue of USD 1557.47 Million in the year 2020 and is further expected to touch USD 5382.17 Million by the end of 2030. One of the major factors anticipated to drive the growth of the market in the region is the increasing awareness for mental health and the rising need amongst healthcare service providers to improve their services to patients who are suffering from the disease. For instance, according to the statistics by the National Alliance on Mental Illness (NAMI), in the United States, in the year 2020, 1 in 5 U.S. adults experiences a mental illness, while 1 in 20 experienced a serious one, and 1 in 15 experienced both a substance use disorder and mental illness. In addition to this, 26.3 Million adults in the nation received virtual mental health services during the COVID-19 pandemic. Amongst those who received these services, 17.7 Million people experienced delays or cancellations in appointments, while 7.3 Million experienced delays in getting prescriptions. Moreover, 4.9 Million people were unable to access needed care. The market in the region is segmented by country into the United States and Canada. Out of the market in these countries, the market in the United States is expected to hold the largest revenue by the end of 2030 and also grow with the highest CAGR of 13.36% during the forecast period.
Request for Research Report Sample-Â https://www.kennethresearch.com/sample-request-10352350
On the other hand, the mental health software and devices market in Europe generated a revenue of USD 1265.71 Million in the year 2020 and is further expected to touch USD 4360.54 Million by the end of 2030, by growing with a CAGR of 13.31% during the forecast period. One of the major factors anticipated to drive the growth of the market in the region is the growing reforms in the diagnosis of mental health. For instance, on the 13th of May, 2019, the Parliamentary Assembly of the Council of Europe (PACE) on Social Affairs, Health, and Sustainable Development, had urged the member States of the Council of Europe to end coercive practices in mental health settings and further adopt a human rights-based approach which is respectful of medical ethics. The market growth in the region can also be attributed to the increasing government initiatives to improve behavioral health services and the surge in the adoption of electronic health records (EHR). The market in the region is further segmented by country into Germany, France, United Kingdom, Italy, Spain, Russia, Netherlands, and the Rest of Europe. Amongst the market in these countries, the market in France is expected to generate the largest revenue of USD 988.54 Million by the end of 2030 and further grow with a CAGR of 13.75% during the forecast period. Additionally, the market in the nation generated a revenue of USD 275.93 Million in the year 2020. Furthermore, the market in Germany registered the second-largest revenue of USD 249.35 Million in the year 2020 and is further expected to touch USD 911.35 Million by the end of 2030.
The study further incorporates Y-O-Y Growth, demand & supply and forecast future opportunity in North America (U.S., Canada), Europe (U.K., Germany, France, Italy, Spain, Hungary, Belgium, Netherlands & Luxembourg, NORDIC [Finland, Sweden, Norway, Denmark], Poland, Turkey, Russia, Rest of Europe), Latin America (Brazil, Mexico, Argentina, Rest of Latin America), Asia-Pacific (China, India, Japan, South Korea, Indonesia, Singapore, Malaysia, Australia, New Zealand, Rest of Asia-Pacific), Middle East and Africa (Israel, GCC [Saudi Arabia, UAE, Bahrain, Kuwait, Qatar, Oman], North Africa, South Africa, Rest of Middle East and Africa).
The global mental health software and devices market is segmented by delivery model into subscription model and ownership model. Amongst these segments, the subscription model segment is projected to garner the largest revenue of USD 2442.34 Million by the end of 2022. Further, the segment is also expected to grow with the highest CAGR of 11.82% during the forecast period. In North America, the segment is expected to garner the highest market share by the end of 2030, while in Europe, the segment is projected to garner the largest revenue of USD 762.37 Million by the end of 2022.
The global mental health software and devices market is further segmented by end user into community clinics, hospitals, private practices, and home care settings. Among these segments, the community clinics segment registered the largest revenue of USD 1644.62 Million in the year 2020 and is further expected to touch a revenue of USD 5667.35 Million by the end of 2030. In North America, the segment generated the largest revenue of USD 660.52 Million in the year 2020 and is further projected to reach USD 2285.27 Million by the end of 2030, by growing with a CAGR of 13.35% during the forecast period. On the other hand, in the Asia Pacific, the segment is anticipated to garner the largest revenue of USD 1124.71 Million by the end of 2030, up from a revenue of USD 317.70 Million in the year 2020. Moreover, in Japan, the segment is expected to garner the largest revenue of USD 244.73 Million and further grow with a CAGR of 14.34% during the forecast period.
The global mental health software and devices market is also segmented on the basis of component, functionality, and mode of access.
Global Mental Health Software and Devices Market, Segmentation by Component
- Software
o Integrated Software
o Standalone Software
- Devices
Global Mental Health Software and Devices Market, Segmentation by Functionality
- Clinical Functionality
o Electronic Health Records (EHR)
o Clinical Decision Support (CDS)
o Care Plans/Health Management
o E-Prescribing
o Telehealth
- Administrative Functionality
o Patient/Client Scheduling
o Document/Image Management
o Case Management
o Workforce Management
o Business Intelligence
- Financial Functionality
o Revenue Cycle Management
o Managed Care
o Accounts Payable/General Ledger
Global Mental Health Software and Devices Market, Segmentation by Mode of Access
- Desktops/Laptops
- Tablets/Smartphone
Some of the prominent industry leaders in the global mental health software and devices market that are included in our report are Cerner Corporation, Netsmart Technologies, Inc., Qualifacts Systems, Inc., Valant, NXGN Management, LLC, Core Solutions, Inc., Holmusk USA, Inc., The Echo Group, Welligent, Inc., Credible Behavioral Health, Inc., IBM, and others.
Request for Research Report Sample-Â https://www.kennethresearch.com/sample-request-10352350
SOURCE: EuropaWire






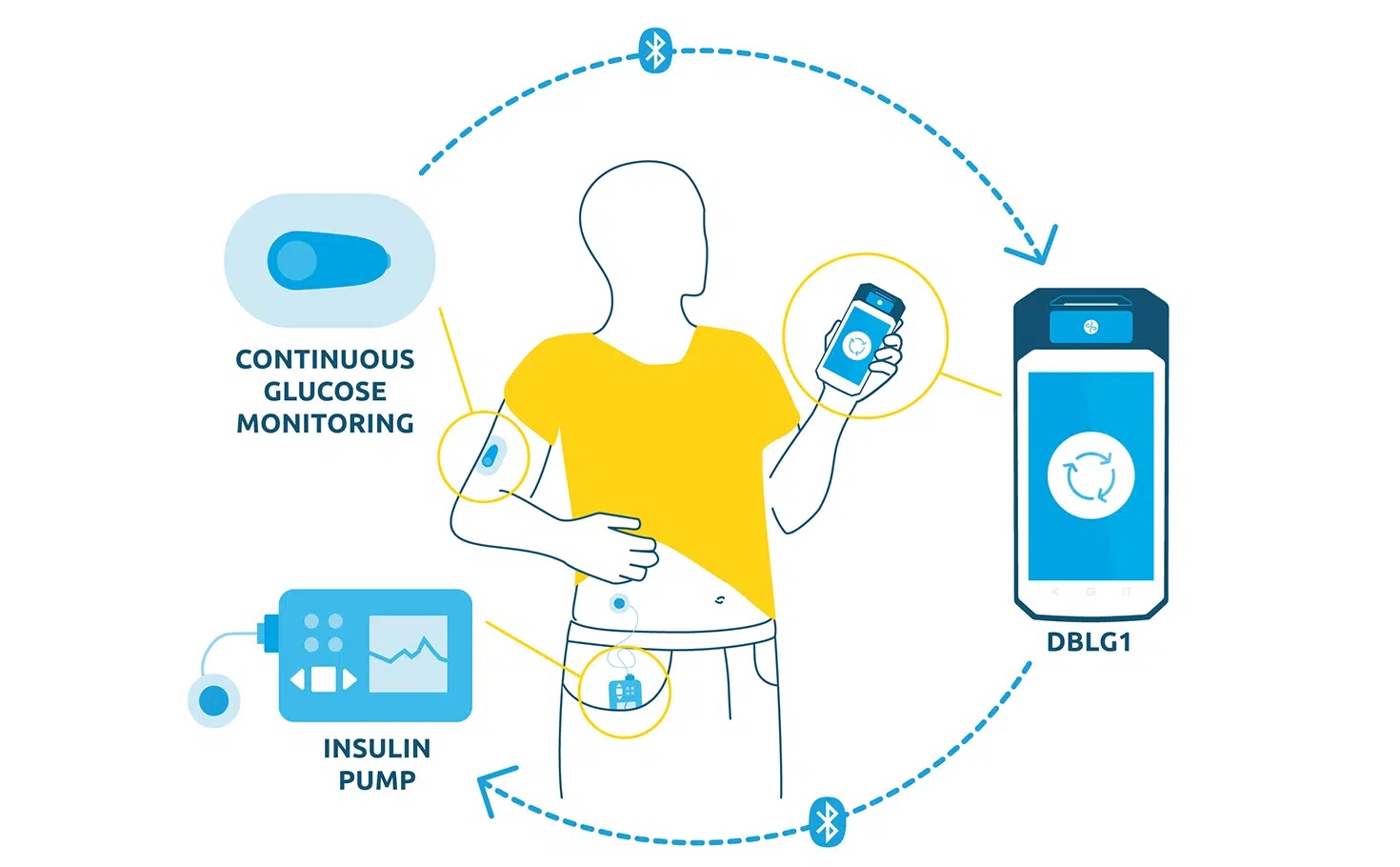
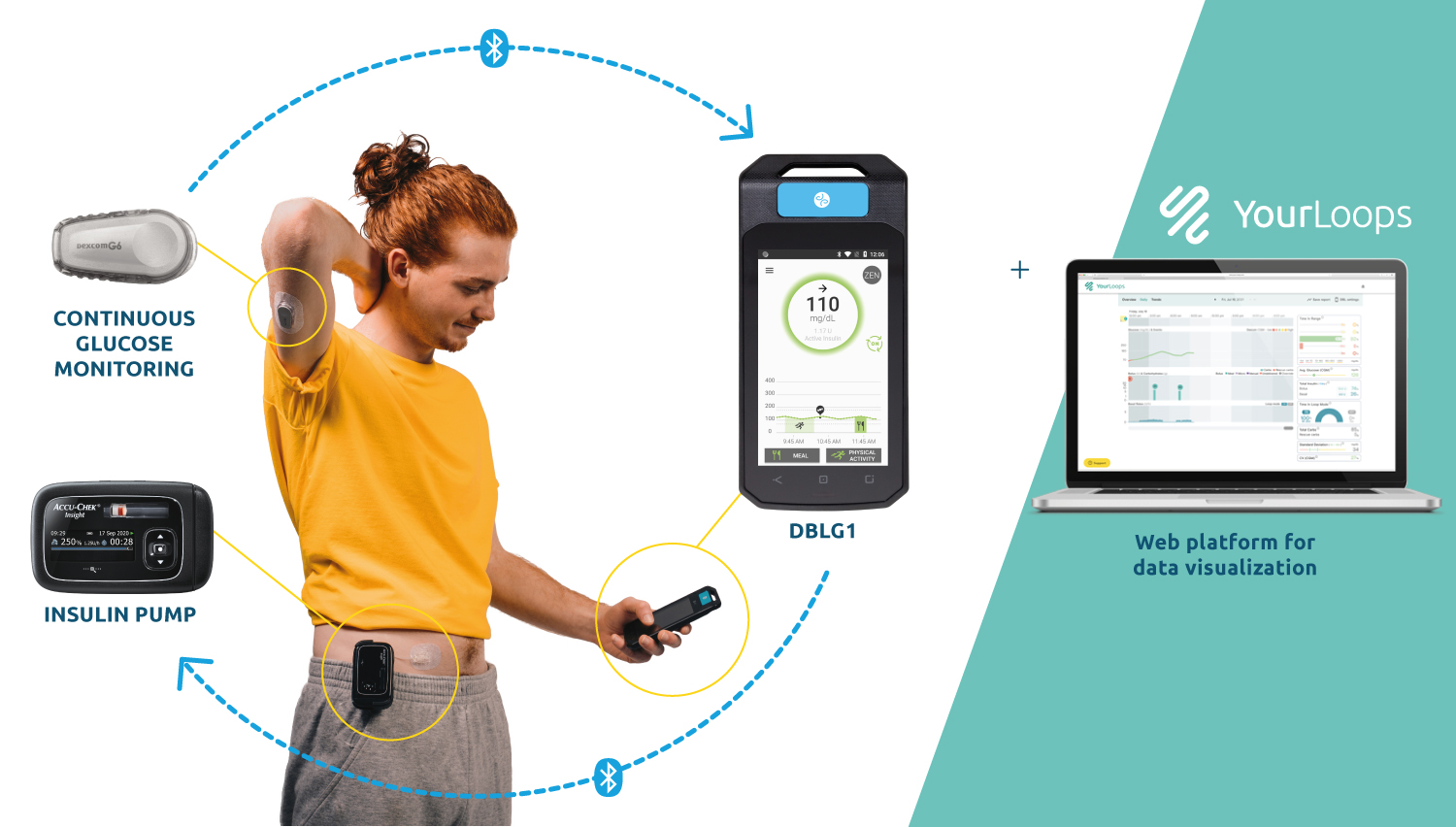
 Listen to all the testimonials in the first episode of the exclusive podcast Diabeloop Stories on Spotify :Â
Listen to all the testimonials in the first episode of the exclusive podcast Diabeloop Stories on Spotify :Â