Baltimore, MD, 2019-Sep-18 — /EPR HEALTHCARE NEWS/ — CoapTech LLC (Baltimore, MD) and Fidmi Medical, Ltd. (Caesarea, Israel) announced today that they will be collaborating on bringing to market a set of next-generation products for the initial placement and long-term maintenance of gastrostomy tubes (G-tubes). The collaboration between CoapTech and Fidmi will focus on integrating the company’s two technologies and expanding their collective market opportunities.
Gastrostomy tubes, which help patients receive long-term nutritional support, are typically placed endoscopically and need to be replaced every 3-6 months.
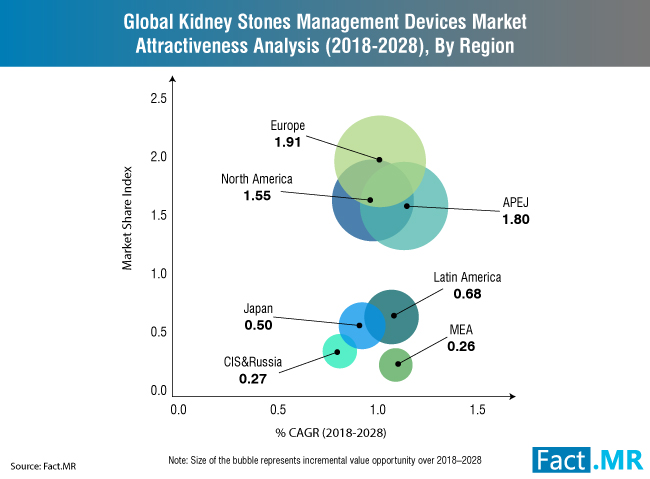
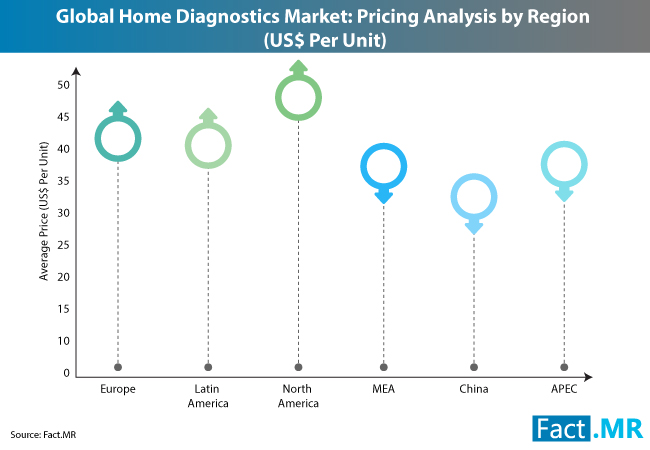
CoapTech’s PUMA-G System allows initial gastrostomy tube placement by non-surgeons/ gastroenterologists, outside of the endoscopy suite, using point-of-care ultrasound at the patient bedside. This novel approach can be performed in the ICU, emergency room, and eventually outside of the hospital setting, substantially reducing costs for tube placement.
Fidmi Medical’s innovative low-profile gastrostomy system is unique in that it can be utilized for both initial placement and replacement and has several features which make it more durable and comfortable for patients. Gastrostomy tubes very often get dislodged, clogged, or infected, and need to be replaced frequently. Fidmi’s improved low-profile gastrostomy tube is placed just like any standard PEG tube but has an easily replaceable inner tube which can be changed by patients without the need to re-enter the healthcare system for replacement procedures. This will result in fewer complications with patients’ g-tubes, therefore potentially reducing healthcare costs for payers and healthcare systems; as well as providing a substantial improvement in quality of life for patients and their caregivers.
Fidmi Medical Chairman, Lloyd Fishman, commented, “We are excited to collaborate with CoapTech in development and marketing. Both companies have developed cutting-edge technologies that will improve patient care and contribute to the ease, efficiency and confidence of clinicians’ work in the gastrostomy field.â€
Dr. Steven Tropello, CoapTech’s founder and CMO said, “In combination these systems will be compatible with Push or Pull methods, be implantable in a wide variety of clinical settings, and require minimal readmission to the hospital and fewer visits to the ER. The synergy will reduce both front and back-end costs for hospitals and payers, and make care safer and more patient-centered.â€
Via EPR Network
More Healthcare press releases